Research
Pomin Lab: BioMolecular NMR and Glycobiology
Research
Dr. Pomin’s group works in the fields of Marine Medicinal Glycomics, NMR-based Structural Glycobiology and Interactomics. The research programs of his laboratory seek to understand the structural and functional details of biomedically active carbohydrates in some pathophysiological events such as coagulation, thrombosis, inflammation, cancer, angiogenesis and microbial infections. His group is expert on the application of NMR technologies on biomolecules. NMR methods are used in great extension for multiple purposes such as structure elucidation of new sugars, assessment of their conformational and dynamical properties in solution and the atomic details in interactions with functional binding proteins. Current investigations focus on the structure-active relationship analysis of sulfated glycans from both terrestrial and marine origins such as glycosaminoglycans, sulfated fucans, and sulfated galactans.
Structure elucidation of novel marine sulfated glycans
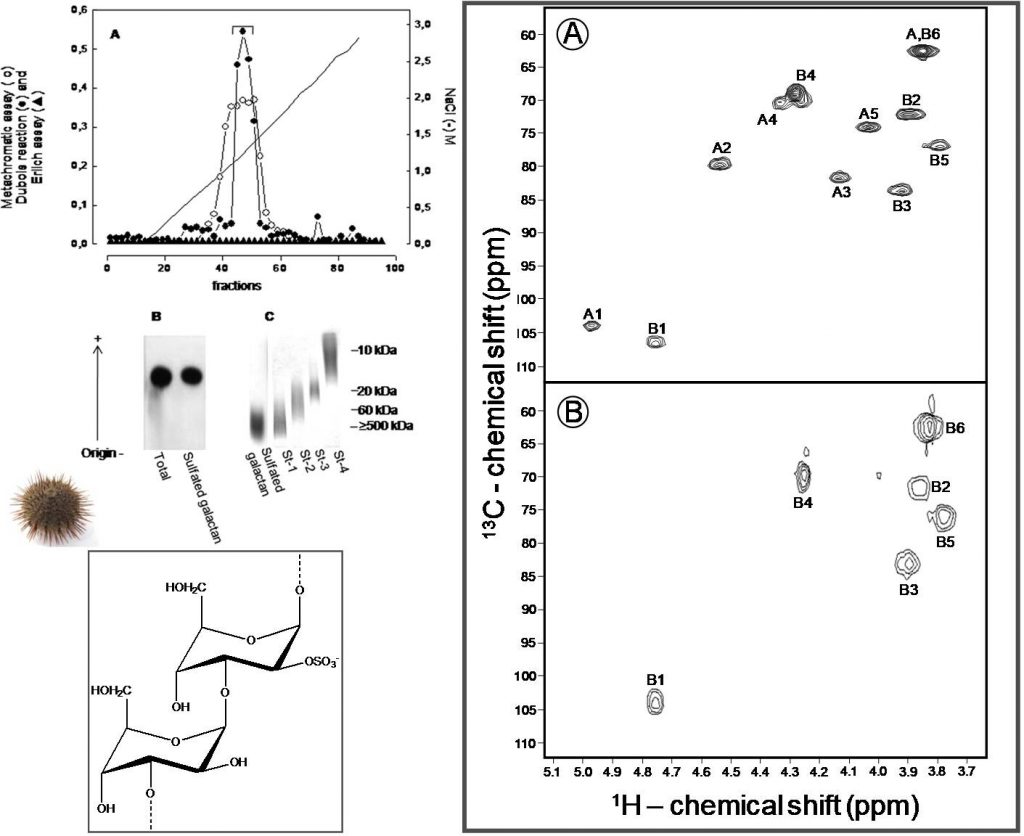
Castro, Pomin VH, Santos LL, Vilela-Silva AC, Hirohashi N, Pol-Fachin L, Verli H, Mourão PA. A unique 2-sulfated {beta}-galactan from the egg jelly of the sea urchin Glyptocidaris crenularis: conformation flexibility versus induction of the sperm acrosome reaction. J Biol Chem. 2009; 284(28):18790-800. doi: 10.1074/jbc.M109.005702.
Comment: In this work, the disaccharide repeating unit of the new sulfated galactan isolated from the sea urchin Glyptocidaris crenularis was elucidated, mainly through NMR methods combined with chemical reactions.
Conformation and dynamics of derived sulfated oligosaccharides
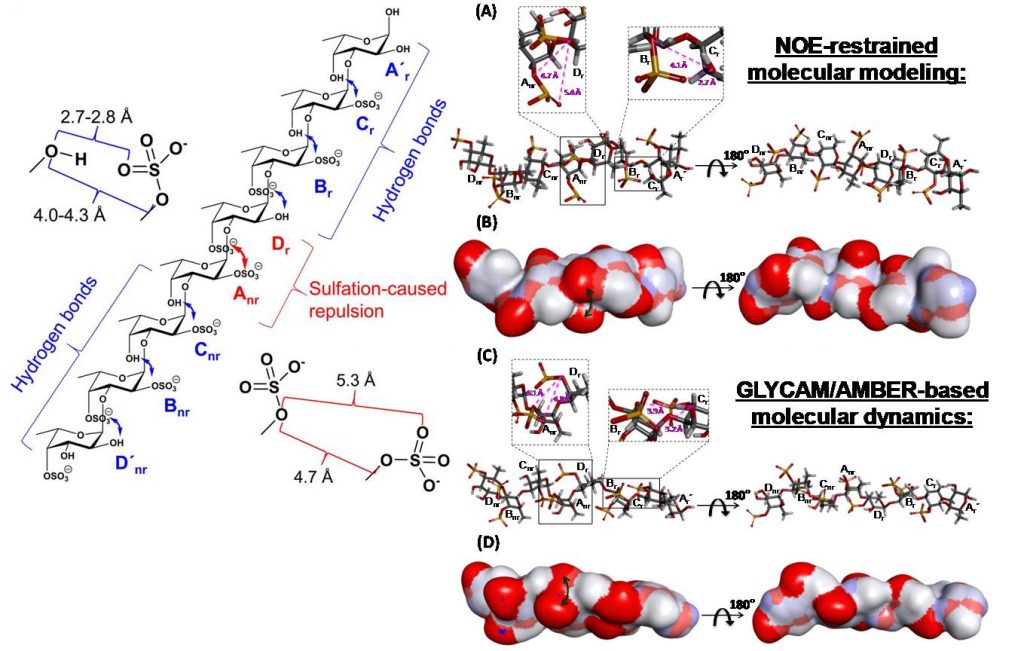
Queiroz IN, Wang X, Glushka JN, Santos GR, Valente AP, Prestegard JH, Woods RJ, Mourão PA, Pomin VH. Impact of sulfation pattern on the conformation and dynamics of sulfated fucan oligosaccharides as revealed by NMR and MD. Glycobiology. 2015; 25(5):535-47. doi: 10.1093/glycob/cwu184.
Comment: In this article, the general aspects related to the 3D structure and dynamics of the octasaccharide derived from the sulfated fucan from the sea urchin Lytechinus variegatus were revealed.
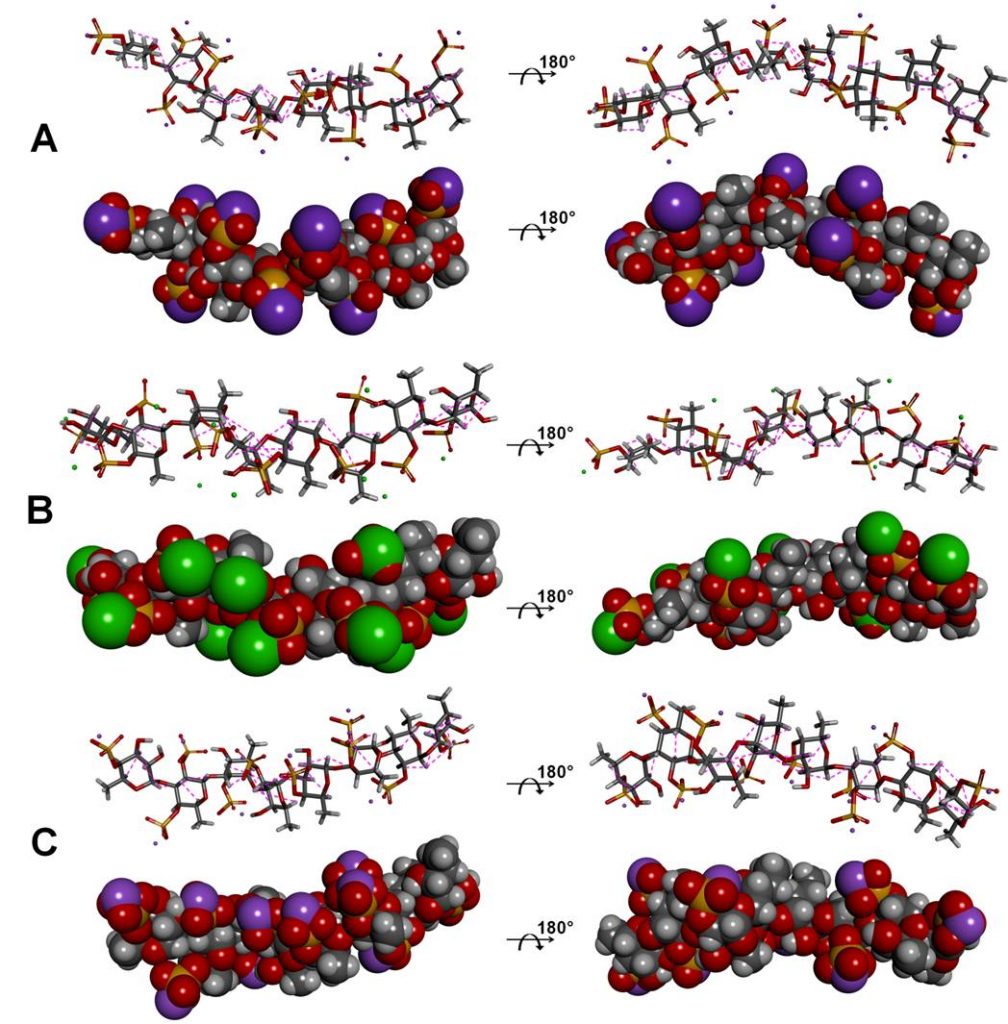
Soares PA, Queiroz IN, Santos GR, Mourão PA, Pomin VH. NMR-based conformation and dynamics of a tetrasaccharide-repeating sulfated fucan substituted by different counterions. Biopolymers. 2016; 105(11):840-51. doi: 10.1002/bip.22922.
Comment: In this article, the conformation and flexibility in solution of the native tetrasaccharide-repeating sulfated fucan from Lytechinus variegatus substituted by three different counterions were investigated.
Molecular Interactions between sulfated glycans and their protein binding partners
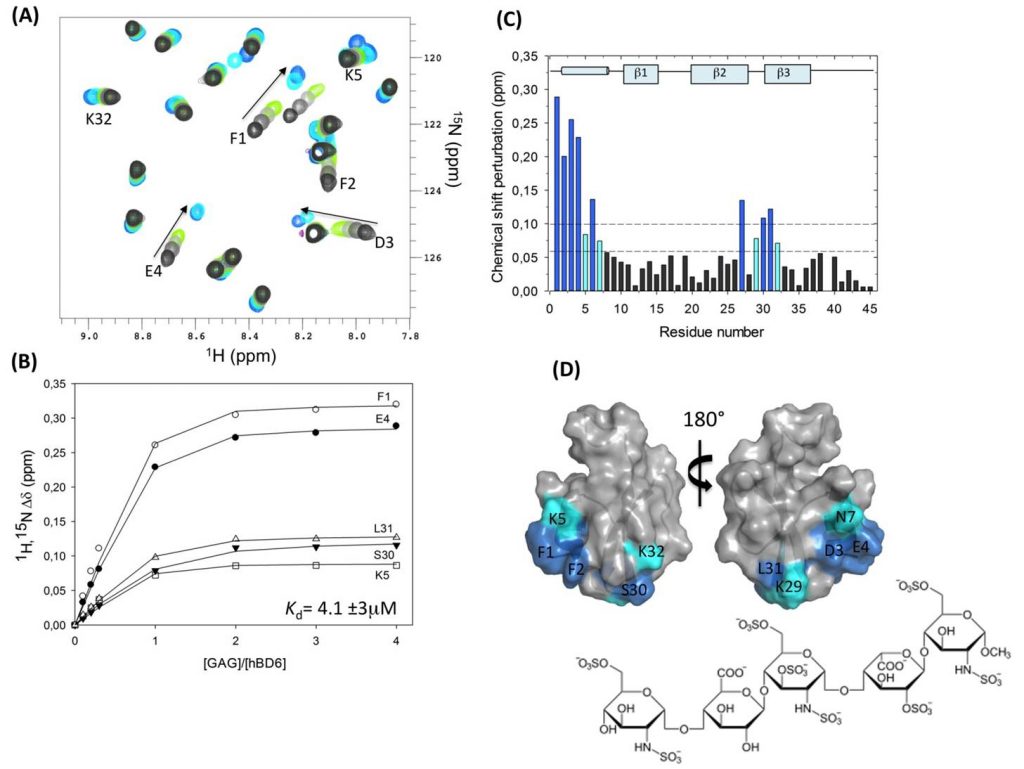
De Paula VS, Pomin VH, Valente AP. Unique properties of human β-defensin 6 (hBD6) and glycosaminoglycan complex: sandwich-like dimerization and competition with the chemokine receptor 2 (CCR2) binding site. J Biol Chem. 2014; 289(33):22969-79. doi: 10.1074/jbc.M114.572529.
Comment: In this publication, the intermolecular complex made between the hBD6 and heparin fondaparinux was investigated at the atomic level by NMR spectroscopy.
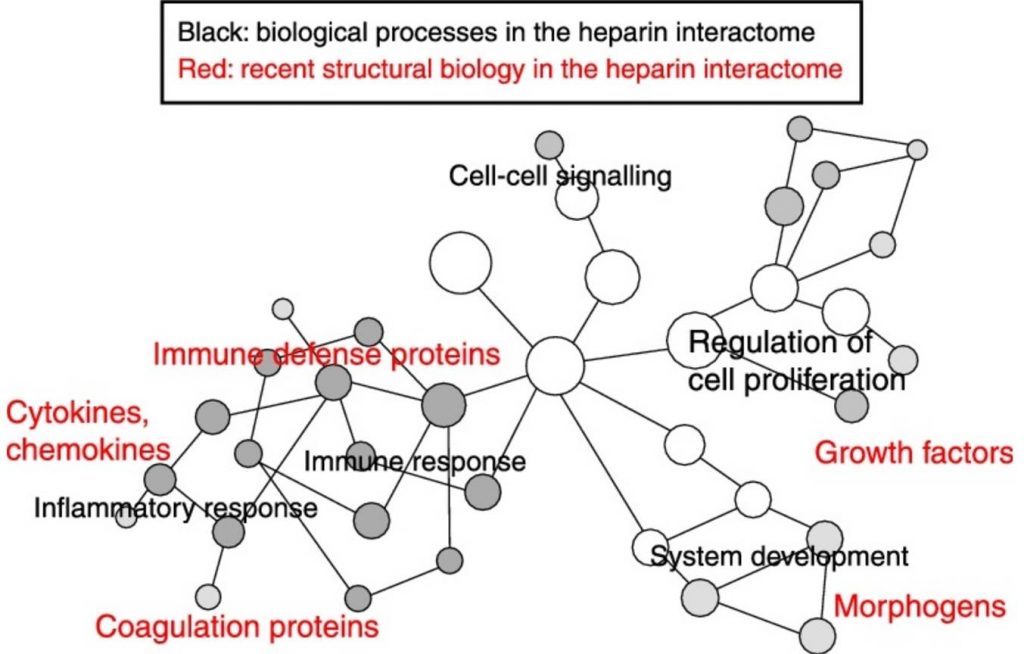
Pomin VH, Mulloy B. Current structural biology of the heparin interactome. Curr Opin Struct Biol. 2015 Oct;34:17-25. doi: 10.1016/j.sbi.2015.05.007. Epub 2015 May 30.
Comment: In this review, the current heparin interactome was systematically analyzed.
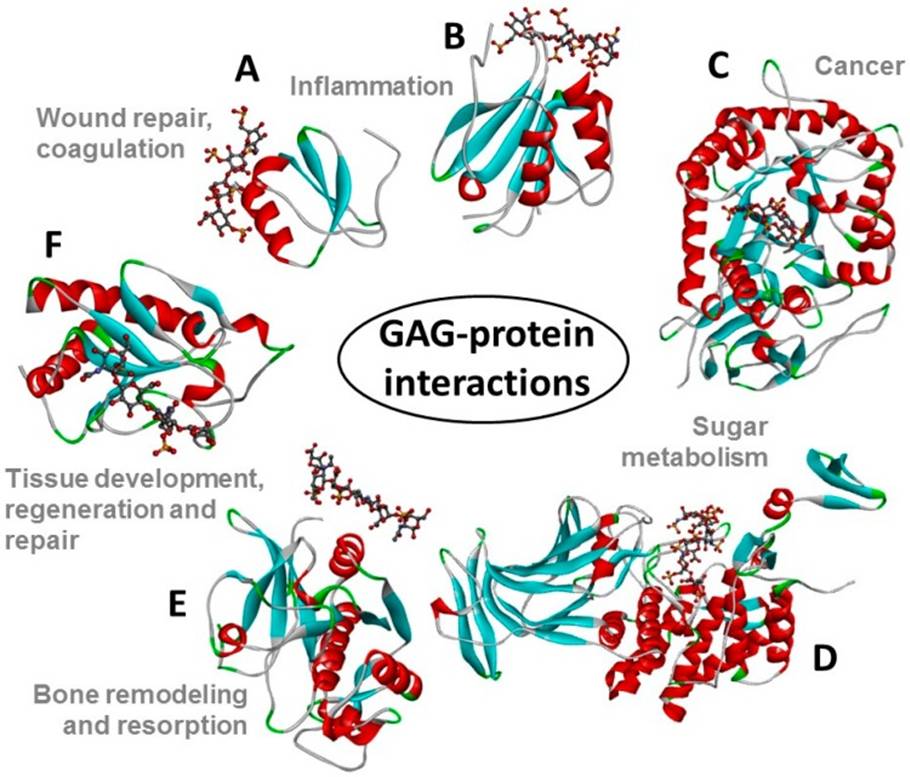
Pomin VH, Wang X. Glycosaminoglycan-Protein Interactions by Nuclear Magnetic Resonance (NMR) Spectroscopy. Molecules. 2018; 23(9). pii: E2314. doi: 10.3390/molecules23092314.
Comment: In this review, the major NMR techniques commonly applied to investigations of glycosaminoglycans-protein interactions is explained.
Structure-function relationship and biological screening of novel marine sulfated glycans
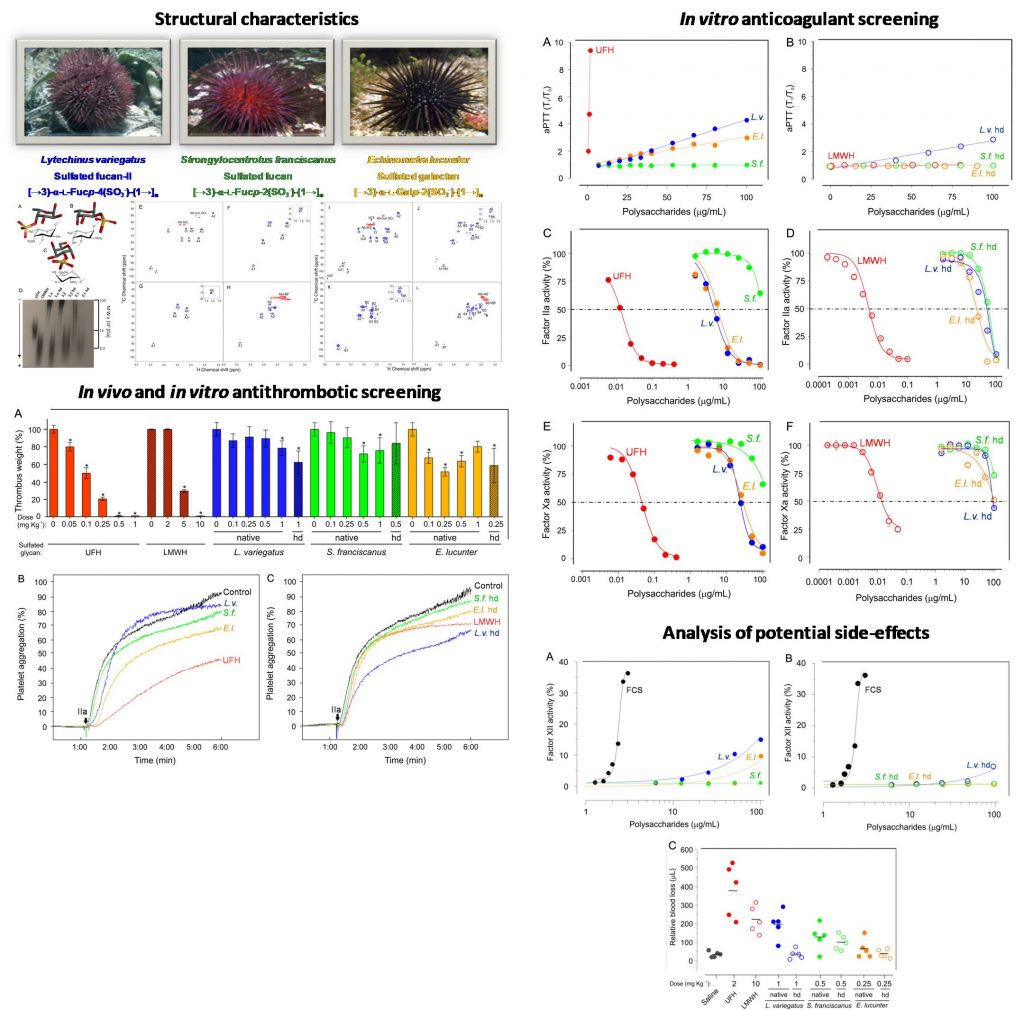
Vasconcelos AA, Sucupira ID, Guedes AL, Queiroz IN, Frattani FS, Fonseca RJ, Pomin VH. Anticoagulant and Antithrombotic Properties of Three Structurally Correlated Sea Urchin Sulfated Glycans and Their Low-Molecular-Weight Derivatives. Mar Drugs. 2018 Aug 30;16(9). pii: E304. doi: 10.3390/md16090304.
Comment: In this article, the structure-activity relationship of three structurally correlated sea urchin-derived sulfated glycans is achieved regarding their potential anticoagulant and antithrombotic potencies.