Jump to:
Immulina
Alasta
Algaecide (NP20851 (DNA2-59-1))
NPC1161B (8-Aminoquinoline)
Immulina™
Immulina™ is a bioassay-standardized extract from the microalgae Spirulina that is used as a dietary supplement for enhancing immune function
Discovery: research was initiated by Drs. David Pasco and Nirmal Pugh in 1997 and continued studies by these and other scientists at the NCNPR and elsewhere led to the development and characterization of this potent extract. The initial discovery of this product occurred when these two scientists developed a sensitive assay for the detection of botanical extracts that enhanced macrophage function. They tested over 50 extracts and products that were traditionally used to enhance immune function and found that extracts from the microalgae Arthrospira (Spirulina) platensis were over 100 times more active than all others tested. The active component (Braun-type lipoproteins) within Immulina™ is present at levels hundreds of times greater than found in other immune-enhancing extracts or herbs.
Bioassay-standardized: the Braun-type lipoproteins in Immulina™ are high molecular weight compounds that are difficult to purify and cannot be standardized using analytical techniques because the physiochemical properties of these molecules do not fully predict their biological activity. Thus, ImmulinaTM is standardized based on units of biological activity. Only Spirulina raw material containing a specified level of activity/potency (NF-kappa B activation in monocytes) is accepted for manufacturing the Immulina™ extract. The criteria for standardization is that each batch of extract must exhibit a minimum level of activity/potency. In addition to using the bioassay to ensure product quality (batch-to-batch consistency), it has also been used to monitor production protocols, formulation of finished products and stability studies.
Stability: No detectable loss in activity/potency of ImmulinaTM during storage at room temperature over a period of 10 years.
Commercialization
Licensed to ChromaDex, Inc. and is available as an ingredient. Immulina™ is protected by two world-wide patent families (US Patent Numbers 7,205,284 and 7,846,452).
Scientific Research
In vitro Studies Animal models Clinical trials
Where can I purchase Immulina™ for individual use?
United States The company Vollara markets a product called Re:Sist that contains ImmulinaTM (branded as ImmuXTTM) as the main ingredient, together with botanical extracts, vitamin C and zinc. Germany The company Dr. Loges markets a product called immunLoges® that contains ImmulinaTM as the main ingredient, together with a fungal beta glucan and basic nutrients.
Scientific research on Immulina™: in vitro studies
The primary target of Immulina™ appears to be the cells of the innate immune system, the body’s first line of defense against invading pathogens.
Research using THP-1 monocytes demonstrates that Immulina™ is a potent activator of NF-kappa B and induced mRNAs encoding IL-1 beta and TNF alpha (Pugh et al, 2001) as well as chemokines IL-8, MCP-1, MIP-1 beta and INF gamma inducible protein-10 (Grazanna et al, 2006).
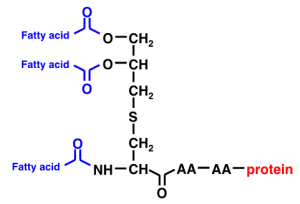
Immulina™ activates monocytes through a Toll-like receptor 2 (TLR2)-dependent pathway (Balachandran et al, 2006). This receptor belongs to a family of receptors that functions to detect components of bacteria, viruses and fungus and thus mediates the initial detection of pathogenic organisms by the immune system.
The major active component within Immulina™ is a class of compounds called Braun-type lipoproteins (Nielsen et al, 2010). These lipoproteins contain a diacylglycerol moiety linked to the N-terminal cysteine of the peptide chain.
References:
Balachandran P, Pugh ND, Ma G, Pasco DS (2006). Int Immunopharmacol 6: 1808-1814.
Grzanna R, Polotsky A, Phan PV, Pugh N, Pasco D, Frondoza CG (2006). J Altern Complement Med 12 (5): 429-435.
Nielsen CH, Balachandran P, Christensen O, Pugh ND, Tamta H, Sufka KJ, Wu X, Walsted A, Schjørring-Thyssen M, Enevold C, Pasco DS (2010). Planta Med 76(16): 1802-1808.
Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS (2001). Planta Med 67 (8): 737-742.
Scientific research on Immulina™: animal models
- Animal research on gut mucosal and systemic immune function
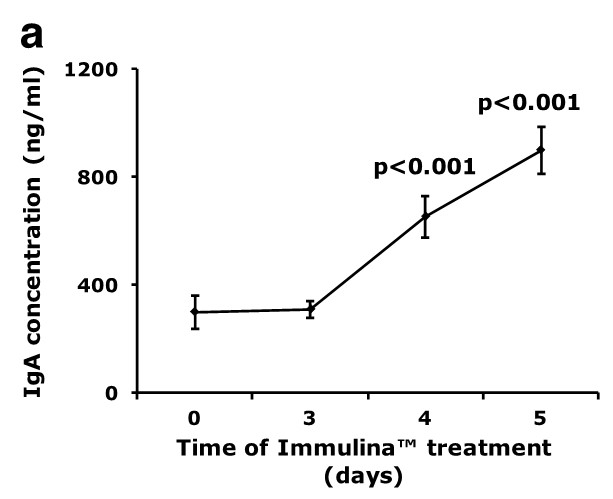
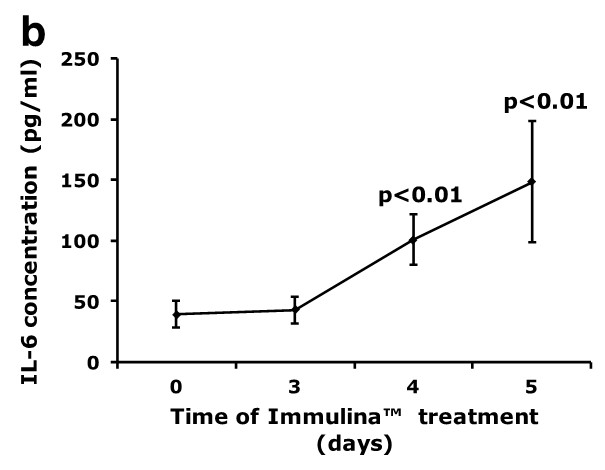
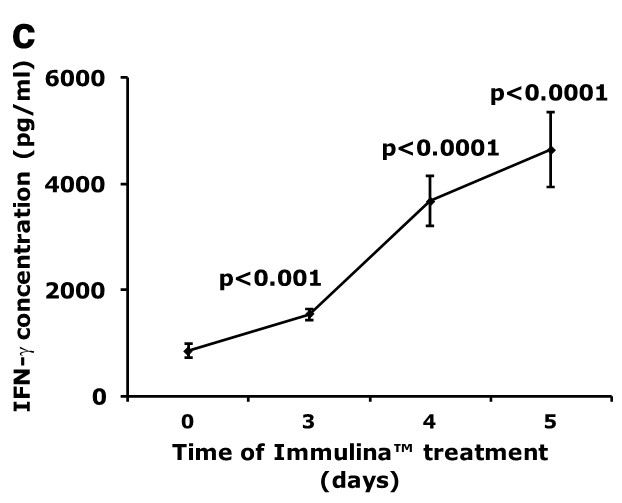
Oral administration of Immulina™ to C3H/He mice enhances the ex vivo production of IgA (Figure a) and IL-6 (Figure b) from Peyer’s patch cells as well as interferon-gamma production (Figure c) from spleen cells. Enhanced IgA production may be involved in mediating the protective effects Immulina™ against viral infection since this antibody prevents the adherence of viruses to mucosal surfaces and is important in eliminating infectious agents.
Balachandran P, Pugh ND, Ma G, Pasco DS (2006). Int Immunopharmacol 6(12): 1808-1814.
2. Animal model for prevention of viral infection
Case studies in Europe suggest that consumption of Immulinaä may enhance resistance to colds and flu. This is supported by a mouse study demonstrating that oral administration of Immulinaä can exhibit a protective effect against influenza A (H1N1) viral infection.
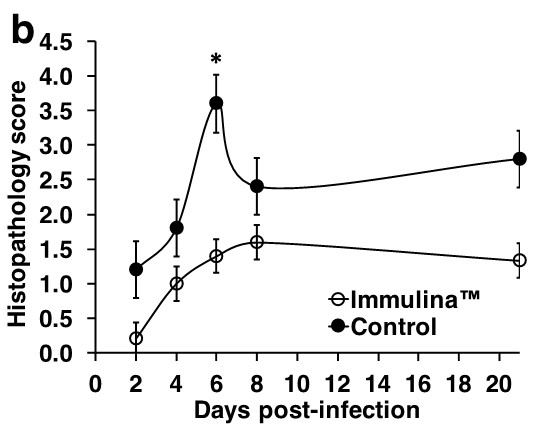
The most dramatic differences observed between treatment and control mice were with respect to weight loss, an indicator of morbidity (Figure a), and lung histopathology, an assessment of damage due to viral replication as well as lymphocyte infiltration (Figure b).
Pugh ND, Edwall D, Lindmark L, Kousoulas KG, Iyer AV, Haron MH, Pasco DS (2015). Phytomedicine 22(2): 271-276.
Scientific research on Immulina™: clinical trials
1. Enhancement of natural killer (NK) cell activity
NK cells play a role in the killing of cancer cells and protection against viral infection. In a pilot study, 10 healthy individuals consuming Immulinaä for one week (400mg/day) showed an average increase of 40% in cancer cell killing by NK cells (p < 0.005, Figure 1).
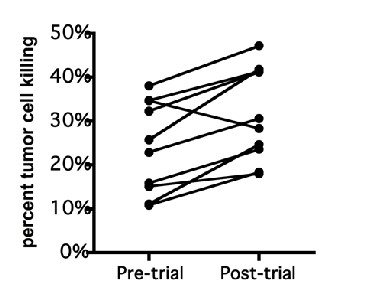
In a separate placebo-controlled, crossover study involving 11 healthy subjects, consumption of Immulinaä for one week (400mg/day) resulted in enhanced mRNA expression of NK cell marker NKG2D by 55% and T-cell marker perforin by 75%.
These results suggest that Immulinaä may be useful for enhancing immune function for prevention of infections and for use in supporting optimal NK cell activity in cancer patients.
Nielsen CH, Balachandran P, Christensen O, Pugh ND, Tamta H, Sufka KJ, Wu X, Walsted A, Schjørring-Thyssen M, Enevold C, Pasco DS (2010). Planta Med 76(16): 1802-1808.
2. Reduction of recurrent HSV-1 (cold sores) outbreak clinical study (unpublished)
In a randomized, double blind, placebo controlled study Immulinaä reduced the number of recurring outbreaks of herpes simplex virus. The endpoint for this study was the number of symptom free individuals (no cold sores). For male subjects, there was a statistically significant reduction in the number of symptom free individuals after 4 months of daily consumption of Immulinaä as compared to placebo. Although female subjects did not show a significant difference in the number of symptom free individuals, the participants consuming Immulinaä did report a reduction in the size and duration of cold sore outbreaks.
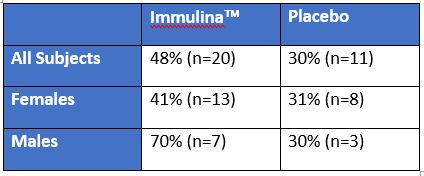
Subjects supplemented their diet with ImmulinaTM (400mg/day) or placebo for 16 weeks. “%” and “n” represents the percentage and number of subjects that were symptom free in weeks 8-16. The ImmulinaTM group contained 42 subjects (10 males, 32 females) and the placebo group had 36 subjects (10 males, 26 females). To be included in the study subjects were required to have more than 6 episodes of cold cores during the previous 12 months. Statistical significance for male subjects was p<0.05 and for all subjects p=0.06, by Fisher’s exact test.
3. Reduction of joint and muscle pain clinical ctudy (unpublished)
A consistent experience by individuals consuming Immulinaä is reduced joint and muscle pain. This reflects more balanced immune function and is supported by a randomized, placebo-controlled study conducted on 103 patients in Denmark over a three month period (400mg/day). Individuals taking Immulinaä exhibited a 40% decrease in pain score and a 52% reduction in the use of pain medication. Mobility scores for these individuals increased by 14%.
4. Enhancement of adaptive immunity clinical study
Eleven healthy male subjects consumed Immulinaä (400mg/day) for 56 days. Ingestion of Immulinaä had an age-dependent and temporary priming effect on the responses of peripheral Th1, Th2 and B cells to antigenic stimuli.
Løbner M, Walsted A, Larsen R, Bendtzen K, Nielsen CH (2008). J Med Food 11(2): 313-322.
Alasta®
Alasta® is a bioassay-standardized Aloe-derived ingredient that is used for enhancing skin elasticity and wrinkle reduction
Discovery: the initial discovery leading to the development of this product occurred in 1998 when Drs. David Pasco and Nirmal Pugh found that the level of macrophage activation exhibited by crude Aloe gel and juice could not be accounted for by known Aloe components such as acemannan. Continued studies by these and other scientists at the NCNPR led to the characterization of a potent immunostimulatory polysaccharide fraction called Aloeride (Pugh et al, 2001) .
Further research at the NCNPR demonstrated that components derived from the naturally occurring bacterial communities within Aloe gel and rind are principal contributors to the innate immune-enhancing properties of this medicinal plant (Debaun et al, 2010). As with essentially all plants, Aloe vera is colonized by bacterial communities. Prior to the NCNPR research, the primary interest in Aloe-associated bacteria was with respect to the quality control of Aloe products. During commercial processing it is important to control potential microbial overgrowth and bacterial contamination from the environment that can result from improper post-harvest processing methods. However, since the leaves of Aloe barbadensis contain naturally occurring bacterial communities, not all Aloe-associated bacteria represent a quality control issue. The most abundant bacteria in the gel are gram-positive cocci that grow well in an acidic environment (pH of aloe gel is 4.5). Gram-positive cocci also colonize the aloe rind (Waller et al, 2004).
The Alasta® ingredient concentrates the level of Aloe-associated bacterial components and the Aloeride polysaccharide to create a product that is a potent activator of innate immune cells. Results from clinical studies on the use of Alasta™ indicates that it balances immune function by reducing overall inflammation in the body and thereby improving skin elasticity and wrinkle reduction.
Pugh N, Ross SA, ElSohly MA, Pasco DS (2001). J Agric Food Chem 49: 1030-1034.
Debaun D, Pasco DS, Pugh NDC, Pashkowsky A, Needleman A (2010). Aloe preparation for skin enhancement. United States Patent Application 20100255130.
Waller TA, Pelley RP, Strickland FM (2004). In Aloes: The genus Aloe; Reynolds T, Ed.; CRC Press: NY, 2004; Chapter 8, pp 139-205.
Bioassay-standardized: the high molecular weight active compounds in AlastaÒ are difficult to purify and cannot be standardized using analytical techniques because the physiochemical properties of these molecules do not fully predict their biological activity. Thus, Alasta® is standardized based on units of biological activity. The criteria for standardization is that each batch of extract must exhibit a minimum level of activity/potency (NF-kappa B activation in monocytes).
Commercialization
Licensed to Woodcliff Skincare Solutions, Inc. and is available as an ingredient.
Intellectual property includes patented (US Patent Numbers 7,196,072 and 7,767,655 ) and patent pending technology (US Application 20100255130) as well as proprietary methods.
Where can I purchase Alasta® for individual use?
The Sustainable Youth® skincare product line contains Alasta® and includes elasticity cream, firming & revitalizing serum, anytime lift eye treatment, super boost night serum and ultra creamy cleansing lotion.
Scientific research on Alasta®: clinical trials
Improved skin elasticity and wrinkle reduction
The impact of Alasta® taken in an oral supplement for skin elasticity was determined by a placebo-controlled, double-blind study on 20 individuals over a 12 week period. Cutometer instrumentation was used to measure and estimate several variables, including elastic, viscoelastic and purely viscous behavior of the skin. In this study, 70% of subjects demonstrated up to 61% improvement in skin elasticity and distensibility. The results were statistically significant and the placebo group showed no significant improvement.
The effect of Alasta® used in a topical application to improve skin elasticity was tested in a placebo-controlled, double-blind study on 30 individuals over a 8 week period. Skin firmness (elasticity) scores were obtained using the Pinch Test method. In this study, 100% of subjects demonstrated up to 72% improvement in skin elasticity over baseline. The results were statistically significant and the placebo group showed no significant improvement.
Debaun D, Pasco DS, Pugh NDC, Pashkowsky A, Needleman A (2010). Aloe preparation for skin enhancement. United States Patent Application 20100255130.
Algaecide (NP20851 (DNA2-59-1)) to control off-flavor causing blue green algae in catfish aquaculture in the United States
Catfish (Ictalurus punctatus) farming is the largest aquaculture industry in the United States. This industry is mainly concentrated in southern states, and more than 50% of the total US catfish production is coming from the state of Mississippi. One of the major problems US catfish farmers are facing is the development of an off-flavor in catfish when the water temperature is above 60o F, rendering them unmarketable. The reason for this problem is the accumulation in catfish flesh of off-flavor-causing secondary metabolites released by microorganisms growing in aquaculture ponds. Off-flavor problems cause harvest delays and financial loss to catfish farmers. Even though several different off-flavors have been identified, the two most common “musty” and “earthy” flavors are caused by the metabolites 2-methylisoborneol (MIB) and geosmin, both produced mainly by blue-green algae. In the southern United States, MIB is responsible for the majority of the off-favor problems in catfish aquaculture, and the blue green algae Osillatoria perornata has been identified as its source. Management of off-flavor causing algae in catfish production ponds is carried out mainly by application of chemicals. Copper-based compounds and the herbicide diuron are the only chemicals currently approved by the EPA for this purpose. Both of these chemicals have major drawbacks, namely their accumulation in the environment and toxicity towards beneficial microorganisms and catfish.
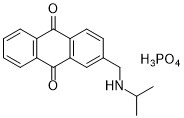
In a program to search for natural product-based algaecides, Dr. Dhammika Nanayakkara of NCNPR and Dr. Kevin K. Schrader of the Natural Products Utilization Research Unit (NPURU) of Agriculture Research Service (ARS) in the US Department of Agriculture (USDA), developed a highly effective and safe alternative, NP20851 (DNA2-59-1), to control O. perornata in catfish production ponds without affecting fish or beneficial microorganisms.
Nanayakkara, N. P. D., Schrader, K. K., (2008) Synthesis of Water-Soluble 9,10-Anthraquinone Analogues with Potent Cyanobactericidal Activity toward the Musty-Odor Cyanobacterium Oscillatoria perornata. J. Agri. Food Chem. 56, 1002-1007.
Schrader, K. K., Nanayakkara, N. P. D., Tucker, C. S., Rimando, A. M., Ganzera, M., Schaneberg, B. T. (2003) Novel derivatives of 9,10-anthraquinone are selective algicides against the musty-odor cyanobacterium Oscillatoria perornata. Appl. Environ. Microbiol. 69, 5319-5327.
Schrader, K K., Foran, C.M., Holmes, B.D., Schlenk, D.K., Nanayakkara, N.P.D., Schanberg, B.T. (2004) Toxicological evaluation of two anthraquinone-based cyanobactericides to channel catfish. N. Am. J. Aquac. 66, 119-124.
Schrader, K.K., Nanayakkara, N.P.D.. (2005), Preparation of 9,10-anthraquinone derivatives as selective algicides for control of blue-green algae in waters. US Patent 6,949,250.
8-Aminoquinoline antiparasitic agents
8-Aminoquinolines are an important class of anti-infective drugs with promising utility in the treatment of malaria, leishmaniasis, and pneumocystis pneumonia. They are the only drugs capable of killing dormant tissue schizonts (hypnozoites) of Plasmodium vivax that cause relapsing malaria. They are also known to kill sporozoites, blood schzonts, and gametocytes, indicating their utility for prophylaxis, treatment, and transmission interruption of malaria. The major drawback of this class of drug is that it causes methemoglobinemia and hemolysis, especially in individuals who are deficient in glucose-6-phosphate dehydrogenase (G6PD).
Primaquine and frintafel (tafenoquine, WR238605) are the only drugs currently approved by the FDA to prevent relapse of P. vivax malaria. Both of these drugs belong to 8-aminoquinoline class, and primaquine has been used for more than fifty years, whereas frintafel was approved in July, 2018. As a precaution to prevent methemoglobineamia and hemolysis in G6PD deficient patients, primaquine is administered in low doses for a long duration. This has led to treatment failures due to poor compliance. On the other hand frintafel could be administered in a single large dose. However, frintafel also can cause hematotoxicity in G6PD deficient patients and is not recommended for use in patients with less than 70% G6PD deficiency.
Both primaquine and frintafel have been approved to be used as racemates. Even though several studies have suggested that enantiomers of this class of compounds may have different activity and toxicity profiles, this phenomenon has received little attention.
For a number of years, NCNPR or the Research Institute of Pharmaceutical Sciences (RIPS) has been studying metabolism, pharmacokinetics, and structure activity relationships of 8-aminoquinolines. A number of 8-aminoquinolines with potent antimalarial activity were resolved into enantiomers, and their biological activity was evaluated. It was found that activity and toxicity of this class of compounds greatly depend on the absolute stereochemistry of drug candidates, and therapeutic utility can be significantly improved by selecting an enantiomer. Out of a number of 8-aminoquinolines drug candidates studied, an enantiomer NPC1161B, was found to have potent activity in treating malaria (prophylactic, therapeutic, and antirelapsing), leishmaniasis, and pneumocystis pneumonia in animal models. These studies showed that the enantiomer NPC1161B is not only consistently more active against the aforementioned parasites, but also less hematotoxic than the other enantiomer or racemate. Currently, efforts are underway to carry out clinical trials on NPC1161B.
An economical method also has been developed to resolve primaquines into enantiomers. These enantiomers have shown comparable radical curative activity in primates, but one enantiomer showed less hematotoxicity than the other enantiomer or racemate in several animal models. Phase I clinical trials of primaquine enantiomers are currently underway at NCNPR to evaluate their relative safety.
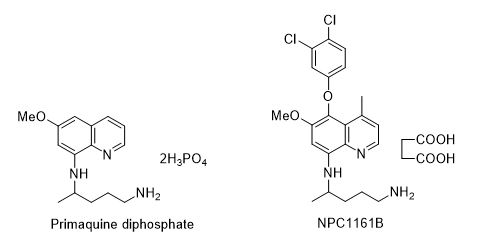
Nanayakkara, N. P. D., Ager Jr., A. L., Bartlett, M. S., Yardley, V., Croft, S. L., Khan, I. A., McChesney, J. D., Walker, L. A. (2008) Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 52, 2130-2137.
Nanayakkara, N. P. D., Tekwani, B. L., Herath, H. M. T. B., Sahu, R., Gettayacamin, M., Tungtaeng, A., van Gessel, Y., Baresel, P., Wickham, K. S., Bartlett, M. S., Fronczek, F. R., Melendez, V., Ohrt, C., Reichard, G. A., McChesney, J. D., Rochford, R., WalkerL. A. (2014) Primaquine Enantiomers: Scalable Preparation and Differential Pharmacologic and Toxicologic Profiles. Antimicrob. Agents Chemother. 58, 4737-4744.
Saunders, D., Vanachayangkul, P., Imerbsin, R., Khemawoot, P., Siripokasupkul, R., Tekwani, B. L., Sampath, A., Nanayakkara, N. P. D., Ohrt, C., Lanteri, C., Gettyacamin, M., Teja-Isavadharm, P., Walker, L.(2014) Pharmacokinetics and pharmacodynamics of (+)-primaquine and (-)-primaquine enantiomers in rhesus macaques (Macaca mulatta). Antimicrob. Agents Chemother. 58, 7283-91.
McChesney, J. D., Nanayakkara, N.P.D., Bartlett, M.S., Ager, A.L. (2002) 8-Aminoquinolines. United States Patent 6,376,511